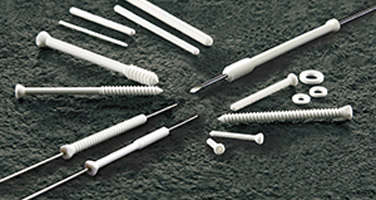
OSTEOTRANS
A high-strength, osteoconductive and bioresorbable material for osteosynthesis.
Teijin Medical Technologies Co., Ltd. has obtained approval for the medical device "OSTEOTRANS", a resorbable bone fixation material composed of bioactive bioceramic microparticles (u-HA) and Poly-L-lactic acid (PLLA) processed in a proprietary method. It possesses bioresorbability, bioactivity, and strength greater than the human cortical bone. This product is manufactured and marketed by our company.
OSTEOTRANS has following characteristics similar to former PLLA products,
- Degrades and resorbs in vivo; therefore, there is no need of removal, thereby reduces patient burden.
- No risk of corrosion, unlike metals.
- It is safe in vivo.
Although research on this type of composite material has been conducted from several perspectives, none of the predicate devices have met the conditions of achieving and maintaining the strength higher than that of the human bone for the minimum period of 3-4 months that are required for bone recovery.
OSTEOTRANS is composed of PLLA and u-HA particles and is molded using our proprietary methods. It succeeds in providing a composite material that is stronger
than the human cortical bone, and maintaining the strength for 4 ~ 6 months. Furthermore, it provides a novel function. After implantation, it is integrated with the surrounding bone and returns to its original state. This function is not available in conventional resorbable materials. The product is manufactured at Yasutomi Factory (Himeji City, Hyogo Prefecture) that has ISO 13485 certification, which is required for quality management systems for medical devices.