About Us
Company History
| 1991 | Takiron Co., Ltd. establishes a medical division to prepare for manufacturing and distributing medical devices. | |
|---|---|---|
| 1993 | The construction of the manufacturing factory for biodegradable/bioresorbable osteosynthesis material at Yasutomi plant completes. |

|
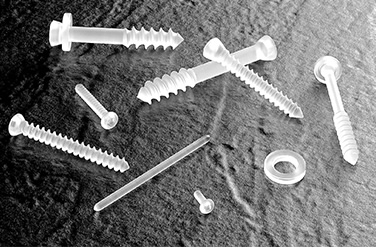
| 1994 | Obtains medical device manufacturing permit. "FIXSORB" is approved in Japan and launched in the orthopedic field. |
|
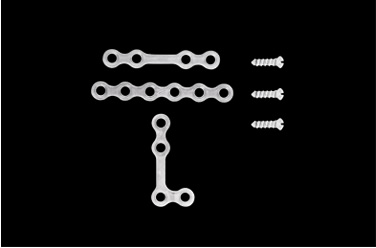
| 1997 | "FIXSORB MX" is approved in Japan and launched in the fields of oral, maxillofacial, plastic, and reconstructive surgery. |
|
| 1999 | Obtains ISO 9001 certification. "FIXSORB MX" is expanded to the field of neurosurgery. |
|
| 2000 | "FIXSORB" and "FIXSORB MX" is approved in China. "FIXSORB rib/sternum pin" is launched in the field of cardiovascular and thoracic surgery. |
|
| 2001 | The construction of the second manufacturing factory at Yasutomi plant completes. | |
| 2002 | Obtains EN ISO 13485 certification. "FIXSORB" is launched in China and expanded overseas. |
|
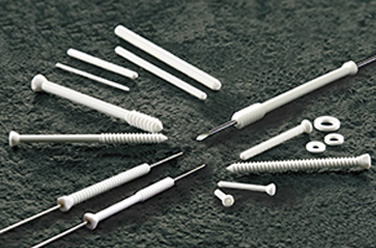
| 2003 | "Super FIXSORB 30" is approved in Japan and launched in the orthopedic field. |
|
| 2004 | "Super FIXSORB 30 rib/sternum pin" is launched in the field of cardiovascular and thoracic surgery. | |
| 2005 | Obtains First class medical device marketing license. CE marking is obtained for "OSTEOTRANS-OT" and "OSTEOTRANS-MX" (overseas trade name). European office opens in Dusseldorf, Germany. |
|
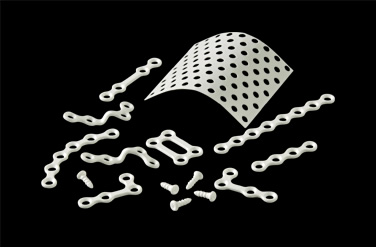
| 2006 | "Super FIXSORB MX30/MX40" is approved in Japan and launched in the field of oral and maxillofacial surgery. A medical institute is established in Kobe City Port Island. |
|
| 2007 | "Super FIXSORB 30 interference screw" is launched in the field of sports medicine. "OSTEOTRANS-OT" is launched in Europe. "FIXSORB MX" is launched in China. |

|
| 2008 | "OSTEOTRANS-OT interference screw" is launched in Europe. | |
| 2009 | "Super FIXSORB MX30/MX40" is expanded to the field of neurosurgery. | |
| 2011 | European office moves to Berlin, Germany. | |
| 2013 | "OSTEOTRANS-MX" is launched in South Korea and Southeast Asia. | |
| 2014 | European office returns to Dusseldorf, Germany. Obtains ISO 13485 certification. |
|
| 2016 | Closes European office. | |
| 2017 | Teijin Limited takes over the medical division of C.I. Takiron Co., Ltd. and establishes Teijin Medical Technologies Co., Ltd. Osaka Headquarter and Tokyo Sales Office opens. Unifies quality assurance standards into ISO 13485. |

|
| 2018 | Medical institute moves to Okayama from Kobe. |

|
| 2019 | "Super FIXSORB EX" is approved in Japan and launched in the field of oral and maxillofacial surgery. |

|
| 2020 | "Super FIXSORB EX" is expanded to the field of neurosurgery. "OSTEOTRANS-MX" is launched in Taiwan. |
|
| 2021 | "GR Tack Pin" (bioresorbable material for alveolar ridge reconstruction) is approved in Japan and launched in the field of dental surgery. |

|
| 2023 | ”SYNFOLIUM” (synthetic cardiovascular patch) is approved in Japan. |

|
| 2024 | Manufacturing factory is established at Teijin Limited Iwakuni plant. Withdrawal from the EU market. ”SYNFOLIUM” is launched in the field of cardiovascular surgery in Japan. |

